

Siklos® Coverage
- Covered at PREFERRED STATUS at a top PBM (covering ~20% of commercial lives)
- Covered WITHOUT RESTRICTIONS on Tricare for patients 18 years & under
- Covered on State Medicaid programs in 37 states. See the Savings section for details


An Innovative Dissolvable Formulation of Hydroxyurea for Patients 2 Years and Older with Sickle Cell Anemia with adaptable administration
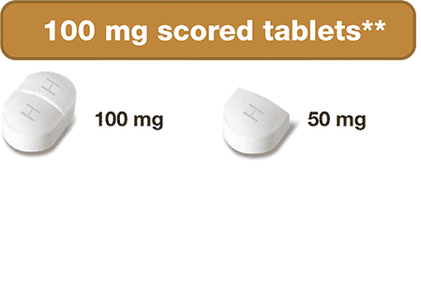
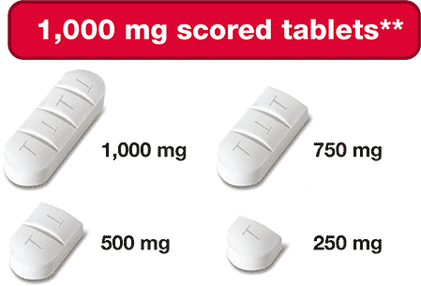 ** Pills are not actual size. † Can be broken into smaller parts
** Pills are not actual size. † Can be broken into smaller parts 
Only with Siklos® do your patients benefit from:

- Dissolvable tablets
- Flexible Dosing & Administration
- Scored & Breakable tablets
- 100 mg & 1,000 mg Strengths
- Conveniently obtained at local pharmacies
- NO compounding
For these reasons, along with its growing commercial and Medicaid coverage, Siklos® had an average prescription increase of ~41% from 2022 to 2024*.
*Based on prescription data from Symphony Health

Siklos® is the FIRST & ONLY FDA-approved dissolvable hydroxyurea-based medication that reduces the frequency of painful crises and the need for blood transfusions in adults and children, 2 years of age and older, with sickle cell anemia with recurrent moderate to severe painful crises.
Siklos® has a BOXED WARNING regarding Myelosuppression and Malignancies.
Serious side effects include embryo-fetal toxicity, cutaneous vasculitic toxicities (including leg ulcers), macrocytosis, and hemolytic anemia.
Most common side effects include infections and neutropenia in children, and infections, headache, and dry skin in adults.
How Siklos® Can Help Patients with Sickle Cell Disease
Siklos® is proven to decrease the frequency of certain SCD events in BOTH children and adults:


Blood Transfusions

Acute Chest Syndrome

Hospitalizations
It is important to treat symptoms of sickle cell disease as early as possible and to continue HU treatment throughout a patients life to reduce the number of SCD events.
How to take Siklos®

The ONLY hydroxyurea medication that can be taken 2 different ways!
- Dissolve in a small amount of water in a teaspoon immediately before use
- Swallow the tablet whole or split based on dose with a glass of water

Siklos® should be taken once daily, at the same time each day, with a glass of water.
Consider starting or switching your appropriate patients to Siklos®
Siklos® is the ONLY hydroxyurea that offers your patients options on how they can take Siklos® - either dissolved in a small amount of water or Siklos® can be swallowed whole or split based on dose
Siklos® tablets must be handled with care. Patients/Caregivers must follow applicable special handling and disposal procedures.
View & print the Instructions for Taking Siklos®

Siklos® is covered by many State Medicaid programs as well as many commercial insurance plans.
Patients with commercial insurance have 2 easy ways to pay as little as $0* for their Siklos® prescription!
Medunik USA also offers savings programs for patients that do not have commercial insurance or if their commercial insurance does not cover Siklos®.
*See Terms and Conditions
INDICATION AND IMPORTANT SAFETY INFORMATION
IMPORTANT SAFETY INFORMATION
WARNING: MYELOSUPPRESSION and MALIGNANCIES
See Full Prescribing Information for complete Boxed Warning.Myelosuppression: SIKLOS may cause severe myelosuppression. Do not give if bone marrow function is markedly depressed. Monitor blood counts at baseline and throughout treatment. Interrupt treatment and reduce dose as necessary.
Malignancies: Hydroxyurea is carcinogenic. Advise sun protection and monitor patients for malignancies.
CONTRAINDICATIONS
SIKLOS is contraindicated in patients who have demonstrated a previous hypersensitivity to hydroxyurea or any other component of its formulation.
WARNINGS AND PRECAUTIONS
Myelosuppression
Hydroxyurea causes severe myelosuppression. Do not initiate treatment with hydroxyurea in patients if bone marrow function is markedly depressed. Bone marrow suppression may occur, and leukopenia is generally its first and most common manifestation. Thrombocytopenia and anemia occur less often and are seldom seen without a preceding leukopenia.
Some patients, treated at the initial dose in adults or in children, have experienced severe or life-threatening myelosuppression. During body weight change modification of daily dose, pediatric patients have an increased risk of myelosuppression at the time of dose adjustment.
Evaluate hematologic status prior to and every two weeks during treatment with SIKLOS. Provide supportive care and modify dose or discontinue SIKLOS as needed. Recovery from myelosuppression is usually observed within 15 days when therapy is interrupted. Resume therapy after interruption at a lower dose.
Malignancies
Hydroxyurea is a human carcinogen. In patients receiving long-term hydroxyurea for myeloproliferative disorders (a condition for which SIKLOS is not approved), secondary leukemia has been reported. Leukemia secondary to long-term hydroxyurea has also been reported in patients with sickle cell disease. Leukemia has also been reported in patients with sickle cell disease and no prior history of treatment with hydroxyurea. Skin cancer has also been reported in patients receiving long-term hydroxyurea. Advise protection from sun exposure and monitor for the development of secondary malignancies.
Embryo-Fetal Toxicity
Based on the mechanism of action and findings in animals, SIKLOS can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus.
Advise females of reproductive potential to use effective contraception during and after treatment with SIKLOS for at least 6 months after therapy. Advise males of reproductive potential to use effective contraception during and after treatment with SIKLOS for at least 6 months after therapy.
Vasculitic Toxicities (including Leg Ulcers)
Cutaneous vasculitic toxicities, including vasculitic ulcerations and gangrene, have occurred in patients with myeloproliferative disorders during therapy with hydroxyurea. These vasculitic toxicities were reported most often in patients with a history of, or currently receiving, interferon therapy. Treatment with SIKLOS should be discontinued and/or its dose reduced if cutaneous vasculitic ulcerations develop. Rarely, ulcers are caused by leukocytoclastic vasculitis. Avoid use of SIKLOS in patients with wounds on the legs (leg ulcers).
Risks with Concomitant Use of Antiretroviral Drugs
Pancreatitis, hepatotoxicity, and peripheral neuropathy have occurred when hydroxyurea was administered concomitantly with antiretroviral drugs. Monitor for signs and symptoms in patients with HIV infection using antiretroviral drugs. Discontinue SIKLOS and implement treatment.
Risks with Concomitant Use of Live Virus Vaccine
Avoid use of live virus vaccine in patients taking SIKLOS as it may potentiate the replication of the vaccine virus and/or may increase the adverse reactions of the vaccine virus and result in severe infection. Patient’s antibody response to vaccines may be decreased. Consider consultation with a specialist.
Macrocytosis
SIKLOS may cause macrocytosis, which is self-limiting, and is often seen early in the course of treatment. The morphologic change resembles pernicious anemia but is not related to vitamin B12 or folic acid deficiency. This may mask the diagnosis of pernicious anemia. Prophylactic administration of folic acid is recommended.
Test Interference
Interference with Uric Acid, Urea, or Lactic Acid Assays is possible, rendering falsely elevated results in patients treated with hydroxyurea.
Interference with Continuous Glucose Monitoring (CGM) Systems is possible, rendering falsely elevated sensor glucose results from certain CGM systems and may lead to hypoglycemia if sensor glucose results are relied upon to dose insulin. If a patient using a CGM is to be prescribed hydroxyurea, consult with the CGM prescriber about alternative glucose monitoring methods.
Hemolytic Anemia
Cases of hemolytic anemia in patients treated with hydroxyurea for myeloproliferative disease have been reported. Monitor blood counts throughout treatment. If hemolysis persists, discontinue SIKLOS.
ADVERSE REACTIONS
The most common adverse reactions to SIKLOS (incidence >10%) include infections and neutropenia in children, and infections, headache, and dry skin in adults.
Other adverse reactions include skin and subcutaneous disorders (skin depigmentation/melanonychia, skin rash, alopecia), gastrointestinal disorders, vitamin D deficiency, and headache.
USE IN SPECIFIC POPULATIONS
Pregnancy
SIKLOS can cause fetal harm. Advise pregnant women of the potential risk to a fetus.
Lactation
Because of the potential for serious adverse reactions in a breastfed child from SIKLOS, including carcinogenicity, advise patients not to breastfeed during treatment with SIKLOS.
Females and Males of Reproductive Potential
Advise patients to inform their healthcare provider of a known or suspected pregnancy. Advise females and males of reproductive potential to use contraception during and after treatment with SIKLOS for at least 6 months after therapy. Based on findings in animals and humans, male fertility may be compromised by treatment with SIKLOS. Prior to therapy, advise male patients about the possibility of sperm conservation.
Pediatric Use
Continuous follow-up of the growth of treated children is recommended. Pediatric patients aged 2-16 years had a higher risk of neutropenia than patients more than 16 years old. The safety and effectiveness of SIKLOS have not been established in pediatric patients less than 2 years of age.
Renal Impairment
Reduce dosage and closely monitor the hematologic parameters when SIKLOS is administered to patients with renal impairment – creatinine clearance of less than 60 mL/min.
Hepatic Impairment
Monitor hematologic parameters more frequently in patients with hepatic impairment receiving SIKLOS.
OVERDOSAGE
Acute mucocutaneous toxicity has been reported in patients receiving hydroxyurea at doses several times above the therapeutic dose. Soreness, violet erythema, edema on palms and soles followed by scaling of hand and feet, severe generalized hyperpigmentation of the skin, and stomatitis have been observed. In patients with sickle cell anemia, neutropenia was reported in isolated cases of hydroxyurea overdose (1.43 – 8.57 times the maximum recommended dose). Treat overdose with gastric lavage, symptom treatment, and control of bone marrow function. Monitor blood counts weekly until recovery.
To report suspected adverse reactions, contact Medunik USA at 1-844-884-5520 or [email protected].
Please read the Full Prescribing Information, including at hcp.SiklosUSA.com.
INDICATION
SIKLOS is an antimetabolite indicated to reduce the frequency of painful crises and to reduce the need for blood transfusions in adult and pediatric patients 2 years of age and older, with sickle cell anemia with recurrent moderate to severe painful crises.
References
- Siklos® (hydroxyurea) tablets, for oral use. [Prescribing Information]. Addmedica.
- Meier ER, Miller JL. Sickle cell disease in children. Drugs. 2012 May 7;72(7):895-906.
- Evidence Based Management of Sickle Cell disease. Expert Panel Report. 2014. NIH. NHLBI.
- Osunkwo I, Andemariam B, Minniti CP, Inusa BPD, El Rassi F, Francis-Gibson B, Nero A, Trimnell C, Abboud MR, Arlet JB, Colombatti R, de Montalembert M, Jain S, Jastaniah W, Nur E, Pita M, DeBonnett L, Ramscar N, Bailey T, Rajkovic-Hooley O, James J. Impact of sickle cell disease on patients' daily lives, symptoms reported, and disease management strategies: Results from the international Sickle Cell World Assessment Survey (SWAY). Am J Hematol. 2021 Apr 1;96(4):404-417. doi: 10.1002/ajh.26063. Epub 2021 Feb 25. PMID: 33264445; PMCID: PMC8248107.
- Yawn BP, John-Sowah, J. Management of Sickle Cell Disease: Recommendations from the 2014 Expert Panel Report. Am Fam Physician. 2015 Dec 15;92(12):1069-76.
- Carvalho, M.; Almeida, I.F. The Role of Pharmaceutical Compounding in Promoting Medication Adherence. Pharmaceuticals 2022, 15, 1091. https://doi.org/10.3390/ph15091091
- USP.org. USP General Chapter 795. “Pharmaceutical Compounding – Nonsterile Preparations.” Accessed September 9, 2021.
- Regulatory Framework for Compounded Preparations. Available from: https://www.ncbi.nlm.nih.gov/books/NBK562888/
- https://packageinserts.bms.com/pi/pi_droxia.pdf
- Xromi (hydroxyurea) 100 mg/mL oral solution [package insert]. Franklin, TN: Rare Disease Therapeutics, Inc.
INDICATION AND IMPORTANT SAFETY INFORMATION
IMPORTANT SAFETY INFORMATION
WARNING: MYELOSUPPRESSION and MALIGNANCIES
See Full Prescribing Information for complete Boxed Warning.Myelosuppression: SIKLOS may cause severe myelosuppression. Do not give if bone marrow function is markedly depressed. Monitor blood counts at baseline and throughout treatment. Interrupt treatment and reduce dose as necessary.
Malignancies: Hydroxyurea is carcinogenic. Advise sun protection and monitor patients for malignancies.
CONTRAINDICATIONS
SIKLOS is contraindicated in patients who have demonstrated a previous hypersensitivity to hydroxyurea or any other component of its formulation.
WARNINGS AND PRECAUTIONS
Myelosuppression
Hydroxyurea causes severe myelosuppression. Do not initiate treatment with hydroxyurea in patients if bone marrow function is markedly depressed. Bone marrow suppression may occur, and leukopenia is generally its first and most common manifestation. Thrombocytopenia and anemia occur less often and are seldom seen without a preceding leukopenia.
Some patients, treated at the initial dose in adults or in children, have experienced severe or life-threatening myelosuppression. During body weight change modification of daily dose, pediatric patients have an increased risk of myelosuppression at the time of dose adjustment.
Evaluate hematologic status prior to and every two weeks during treatment with SIKLOS. Provide supportive care and modify dose or discontinue SIKLOS as needed. Recovery from myelosuppression is usually observed within 15 days when therapy is interrupted. Resume therapy after interruption at a lower dose.
Malignancies
Hydroxyurea is a human carcinogen. In patients receiving long-term hydroxyurea for myeloproliferative disorders (a condition for which SIKLOS is not approved), secondary leukemia has been reported. Leukemia secondary to long-term hydroxyurea has also been reported in patients with sickle cell disease. Leukemia has also been reported in patients with sickle cell disease and no prior history of treatment with hydroxyurea. Skin cancer has also been reported in patients receiving long-term hydroxyurea. Advise protection from sun exposure and monitor for the development of secondary malignancies.
Embryo-Fetal Toxicity
Based on the mechanism of action and findings in animals, SIKLOS can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus.
Advise females of reproductive potential to use effective contraception during and after treatment with SIKLOS for at least 6 months after therapy. Advise males of reproductive potential to use effective contraception during and after treatment with SIKLOS for at least 6 months after therapy.
Vasculitic Toxicities (including Leg Ulcers)
Cutaneous vasculitic toxicities, including vasculitic ulcerations and gangrene, have occurred in patients with myeloproliferative disorders during therapy with hydroxyurea. These vasculitic toxicities were reported most often in patients with a history of, or currently receiving, interferon therapy. Treatment with SIKLOS should be discontinued and/or its dose reduced if cutaneous vasculitic ulcerations develop. Rarely, ulcers are caused by leukocytoclastic vasculitis. Avoid use of SIKLOS in patients with wounds on the legs (leg ulcers).
Risks with Concomitant Use of Antiretroviral Drugs
Pancreatitis, hepatotoxicity, and peripheral neuropathy have occurred when hydroxyurea was administered concomitantly with antiretroviral drugs. Monitor for signs and symptoms in patients with HIV infection using antiretroviral drugs. Discontinue SIKLOS and implement treatment.
Risks with Concomitant Use of Live Virus Vaccine
Avoid use of live virus vaccine in patients taking SIKLOS as it may potentiate the replication of the vaccine virus and/or may increase the adverse reactions of the vaccine virus and result in severe infection. Patient’s antibody response to vaccines may be decreased. Consider consultation with a specialist.
Macrocytosis
SIKLOS may cause macrocytosis, which is self-limiting, and is often seen early in the course of treatment. The morphologic change resembles pernicious anemia but is not related to vitamin B12 or folic acid deficiency. This may mask the diagnosis of pernicious anemia. Prophylactic administration of folic acid is recommended.
Test Interference
Interference with Uric Acid, Urea, or Lactic Acid Assays is possible, rendering falsely elevated results in patients treated with hydroxyurea.
Interference with Continuous Glucose Monitoring (CGM) Systems is possible, rendering falsely elevated sensor glucose results from certain CGM systems and may lead to hypoglycemia if sensor glucose results are relied upon to dose insulin. If a patient using a CGM is to be prescribed hydroxyurea, consult with the CGM prescriber about alternative glucose monitoring methods.
Hemolytic Anemia
Cases of hemolytic anemia in patients treated with hydroxyurea for myeloproliferative disease have been reported. Monitor blood counts throughout treatment. If hemolysis persists, discontinue SIKLOS.
ADVERSE REACTIONS
The most common adverse reactions to SIKLOS (incidence >10%) include infections and neutropenia in children, and infections, headache, and dry skin in adults.
Other adverse reactions include skin and subcutaneous disorders (skin depigmentation/melanonychia, skin rash, alopecia), gastrointestinal disorders, vitamin D deficiency, and headache.
USE IN SPECIFIC POPULATIONS
Pregnancy
SIKLOS can cause fetal harm. Advise pregnant women of the potential risk to a fetus.
Lactation
Because of the potential for serious adverse reactions in a breastfed child from SIKLOS, including carcinogenicity, advise patients not to breastfeed during treatment with SIKLOS.
Females and Males of Reproductive Potential
Advise patients to inform their healthcare provider of a known or suspected pregnancy. Advise females and males of reproductive potential to use contraception during and after treatment with SIKLOS for at least 6 months after therapy. Based on findings in animals and humans, male fertility may be compromised by treatment with SIKLOS. Prior to therapy, advise male patients about the possibility of sperm conservation.
Pediatric Use
Continuous follow-up of the growth of treated children is recommended. Pediatric patients aged 2-16 years had a higher risk of neutropenia than patients more than 16 years old. The safety and effectiveness of SIKLOS have not been established in pediatric patients less than 2 years of age.
Renal Impairment
Reduce dosage and closely monitor the hematologic parameters when SIKLOS is administered to patients with renal impairment – creatinine clearance of less than 60 mL/min.
Hepatic Impairment
Monitor hematologic parameters more frequently in patients with hepatic impairment receiving SIKLOS.
OVERDOSAGE
Acute mucocutaneous toxicity has been reported in patients receiving hydroxyurea at doses several times above the therapeutic dose. Soreness, violet erythema, edema on palms and soles followed by scaling of hand and feet, severe generalized hyperpigmentation of the skin, and stomatitis have been observed. In patients with sickle cell anemia, neutropenia was reported in isolated cases of hydroxyurea overdose (1.43 – 8.57 times the maximum recommended dose). Treat overdose with gastric lavage, symptom treatment, and control of bone marrow function. Monitor blood counts weekly until recovery.
To report suspected adverse reactions, contact Medunik USA at 1-844-884-5520 or [email protected].
Please read the Full Prescribing Information, including at hcp.SiklosUSA.com.
INDICATION
SIKLOS is an antimetabolite indicated to reduce the frequency of painful crises and to reduce the need for blood transfusions in adult and pediatric patients 2 years of age and older, with sickle cell anemia with recurrent moderate to severe painful crises.